ANNUAL
REPORT
April 16, 2001
Dear
Stockholder,
This
past year has been another record one for us, with both sales
and earnings setting new highs.
At $.42 per share our earnings this year increased 50%
over the $.28 of the previous year, and revenue was up 14%.
Sales of the Guardian Laboratories division, which
accounts for approximately 85% of the company’s revenues,
were up 22%.
For the first time revenue exceeded $10 million, and
earnings after taxes exceeded $2 million.
The following charts graphically illustrate the steady growth
we have experienced over the past few years:
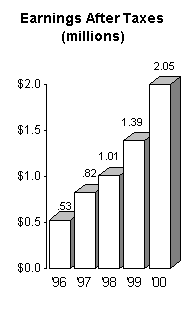 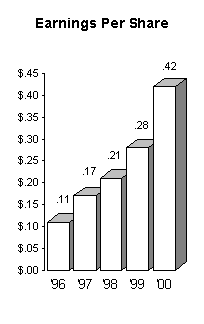
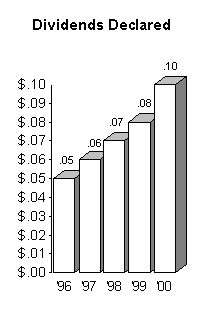
In
addition to the steady increases in revenue and earnings, we
have also continued to strengthen our balance sheet, with a
current ratio (current assets vs. current liabilities) of 7.8
to 1, up from 6.9 to 1 in 1999.
We continue to be virtually debt free while maintaining
a $700,000 line of credit with our main bank in the event
additional funding is ever needed.
Our cost of sales as a percentage of sales decreased to
48% in 2000 from 54% in 1999 as we were able to increase our
production while keeping our overhead down.
In
July 2000 we entered into an expanded marketing agreement with
ISP, which markets our personal care products in the
U.S.
,
Canada
,
Mexico
, South and
Central
America
,
parts of
Europe
and most of
Asia
. This new agreement replaces two previous
marketing agreements we had in place with ISP for domestic and
foreign distribution of our products, and expands ISP’s
marketing efforts into new territories. ISP’s sales of our
products increased substantially in the past year, with some
of that increase being attributable to rapidly growing sales
of our core products in Mainland
China
. Our customers in
China
now number in the hundreds, and their requirements
for our products grew steadily over this past year. We
also saw a significant increase in sales in
Europe
, particularly in
France
through our French distributor Sederma, a
subsidiary of Croda International.
Most
of the growth in our sales was attributable to our Lubrajel®
line of water-based moisturizing gels, which is our most
important product line.
We expect Lubrajel sales to continue to grow not only
in the cosmetic industry, but for medical uses as well as we
continue to develop new markets for this product line.
While
we are very pleased that our Lubrajel sales have continued to
increase, we are not just relying on that product line to
generate our future growth. This past year saw the
introduction of Confetti™ II Dermal Delivery Flakes, the
successor to our original Confetti Dermal Essentials product.
Confetti is a proprietary product line that incorporates
various oil soluble ingredients into colorful flakes that can
be suspended in clear water-based formulations to create
products that retain their clarity and are both colorful and
functional. The original Confetti had inherent
limitations that prevented it from being used in certain
formulations. Confetti II overcomes most of these
limitations, allowing the product to be used in a much wider
variety of formulations.
We are continuing to work with a major personal care products
company headquartered in the
United Kingdom
that is in the process of substituting Lubrajel
Fluid, a special grade of Lubrajel made specifically for them,
into one of their globally marketed personal care product
lines. We have
been working with them steadily over the past few years to
fine-tune the formula so that it would be compatible with all
of the product lines into which they would like to incorporate
our product. We
believe that we have now achieved that goal.
While we previously shipped product to them for
incorporation into product earmarked for parts of
Europe
, we are now in the process of shipping the final
formulation to four of their locations in different parts of
the world for testing and pilot runs.
We are hopeful that by the end of this year they will
begin full-scale production using our product on a worldwide
scale.
As
you may recall, we test marketed our Razoride™ shaving gel
on the Internet last year, and received some very favorable
comments from some of the customers who tried it.
Razoride is a non-foaming gel that has unique
lubricating properties, excellent after-feel, and, because it
contains no soaps, solvents, or dyes, is hypoallergenic. We
are now working in several areas to see if we can develop a
market for this product. We
have entered into a contract with a company that places
product with all of the major catalog companies.
They will be developing promotional materials for us
and submitting them to the appropriate catalogs.
There will be no cost to us for their efforts unless
the product is placed and sales occur.
We are also pursuing leads generated for us by an
outside business consulting firm, The Kline Group.
They generated a list and made initial contacts with
several companies across the
U.S.
that expressed some interest in marketing the
product. We are
now following up on those leads.
We are also continuing to sell Razoride in bulk to two
companies selling the product for special non-consumer uses,
and we have developed a new non-alcohol based skin sanitizer
for one of those companies.
We are hopeful that these products may be just the
first of many products we may be developing for this company.
In
the medical area we have contracted with
Boston
University
to perform some preliminary clinical trials on the
use of Clorpactin®, our powerful chlorine-based
antimicrobial, for the treatment of gingivitis.
The researchers at Boston University are in the process
of obtaining approval from their Institutional Review Board to
proceed with the project, and once that happens we expect to
have results in a couple of months.
If the tests are positive we will then decide whether
we are going to proceed with further clinical trials on our
own, or use those results to locate a partner to work with us
to obtain regulatory approval for the treatment of gingivitis,
as well as possibly expanding the uses to other periodontal
diseases.
In
regard to our work with Sonarite®, we are
continuing our efforts to develop a formulation that is as
effective as our original Sonarite formulation.
This product was originally developed by us as a
treatment for snoring, and a preliminary clinical study showed
that it had some efficacy in treating mild to moderate sleep
apnea as well. Unfortunately
that original formulation can no longer be produced due to the
discontinuation of one of the key raw materials, and we have
been trying to find a suitable substitute ever since.
Until we develop a new formulation that is as effective
as the original one we will not be able to proceed further
with this project.
We are continuing our efforts to streamline our
Eastern Chemical subsidiary by reducing inventory levels.
This has been an ongoing process over the past few
years, and our ultimate goal is to have the Eastern inventory
consist only of fast moving items to make it more marketable
in the event we once again decide to actively look for a buyer
for that operation. Since
inventory levels were the major obstacle to a sale of Eastern
when we looked into this a few years ago, by reducing
inventory we believe that we will have a much greater chance
of selling the operation if and when the time comes to do so.
As
most of you probably know by now, in October we were notified
by Forbes Magazine that we had been chosen one of the “200
BEST SMALL COMPANIES IN
AMERICA
”. This
is the first time our company has appeared on this list, and
we believe that it is an indication of the excellent progress
we have made over the past few years in increasing revenue,
earnings, and financial strength.
To be eligible for inclusion on this list a company
needed to have sales of between $5 million and $350 million,
at least a 5% annual growth in revenue and earnings-per-share
over the past five years, and total net income in excess of $1
million over the past four fiscal quarters.
United-Guardian was 120th overall on the list, but were
78th for earnings growth rate over the past five years, which
averaged 40% per year. As
a result of our excellent financial results over this past
year this earnings growth figure will now be even higher, and
we are hopeful that we will be included on the
Forbes
list again this year.
We
are very pleased with the progress we have made in increasing
our earnings while keeping our costs in check.
We are continuing to expand the marketing of our
products while endeavoring to streamline our operations even
further. With the
many projects we are working on we expect to continue our
growth in revenue and earnings with minimal increases in
operating costs. Since
most of our earnings growth has come from increases in sales
of our existing core products, as some of our new projects
come to fruition we are well positioned to increase our sales
significantly. We
are very excited about our growth potential over the next few
years, and are confident that the steady growth we have
experienced over the past seven years will continue.
Sincerely,
Dr.
Alfred
R.
Globus
Chairman and CEO
|









